Ocean acidification is a largely forgotten consequence of climate change. It is certainly one of the less publicized issues. Hurricanes, droughts, heatwavesor floods all make for great headlines, with spectacular images and important human losses. Even climate deniers have not invented some controversy about ocean acidification, which shows how little it is in the public eye.
There is, however, a lot at stake. The food web, biodiversity loss and, more generally, life in several regions of the world are threatened by ocean acidification. There is no planet B, therefore there is no ocean B. That’s probably what plankton, fish and corals would say, given that they are all threatened by extinction because of the combined effects of ocean acidification and other consequences of climate change… Unfortunately, they have no chance to speak out…
What role does – and will – climate change play in ocean acidification ? What are the consequences, and who is affected ? Will France be spared by this phenomenon? How can we avoid it?
Jean-Pierre Gattuso, a scientist at the CNRS, working at the Laboratory of Oceanography of Villefranche-sur-Mer, helped us to answer these questions.
What is ocean acidification ?
The IPCC defines ocean acidification as “A reduction in the pH of the ocean, accompanied by other chemical changes, over an extended period, typically decades or longer, which is caused primarily by uptake of carbon dioxide (CO2) from the atmosphere, but can also be caused by other chemical additions or subtractions from the ocean. Anthropogenic acidification refers to the component of pH reduction that is caused by human activity“.
Every year, the ocean and the atmosphere exchange large quantities of CO2 with the atmosphere. Since the eighties, the oceans have absorbed over 25% of all anthropogenic CO2 emissions – around 40 billion metric tons of CO2 – from the atmosphere each year. Without this CO2 intake global warming would be much worse than it is today. However, it’s bad news for the ocean, as it contributes to its acidification.
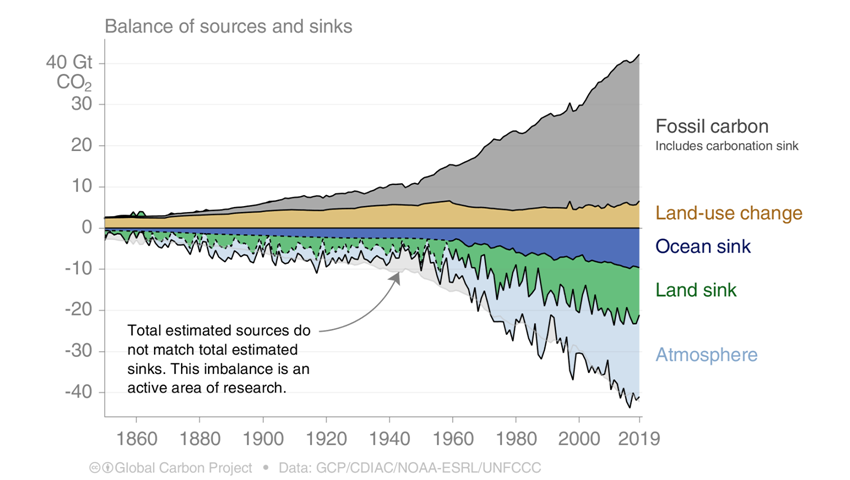
Source : Global Carbon Projet
When CO2 dissolves in sea water a series of chemical reactions occur, thereby decreasing the ocean’s pH, leading to ocean acidification. Just a reminder, the pH scale goes from 0 to 14, with 7 being neutral. Anything higher than 7 is basic (or alkaline) and anything lower than 7 is acidic. Here’s a quick chemistry lesson to help understand acidification:
As carbon dioxide (CO2) dissolves in sea water, it forms carbonic acid (H2CO3), a weak acid that breaks (or “dissociates”) into bicarbonate ions (HCO3–) and hydrogen ions (H+). A higher level of H+ ions increases acidity (a decrease in pH). But that’s not the only problem. Ocean acidification is slowed down because available carbonate ions (CO32-) bond with excess hydrogen (H+), which forms bicarbonate. However, this buffering effect consumes CO32- and thereby reduces the chemical capacity of the upper ocean to absorb more CO2.
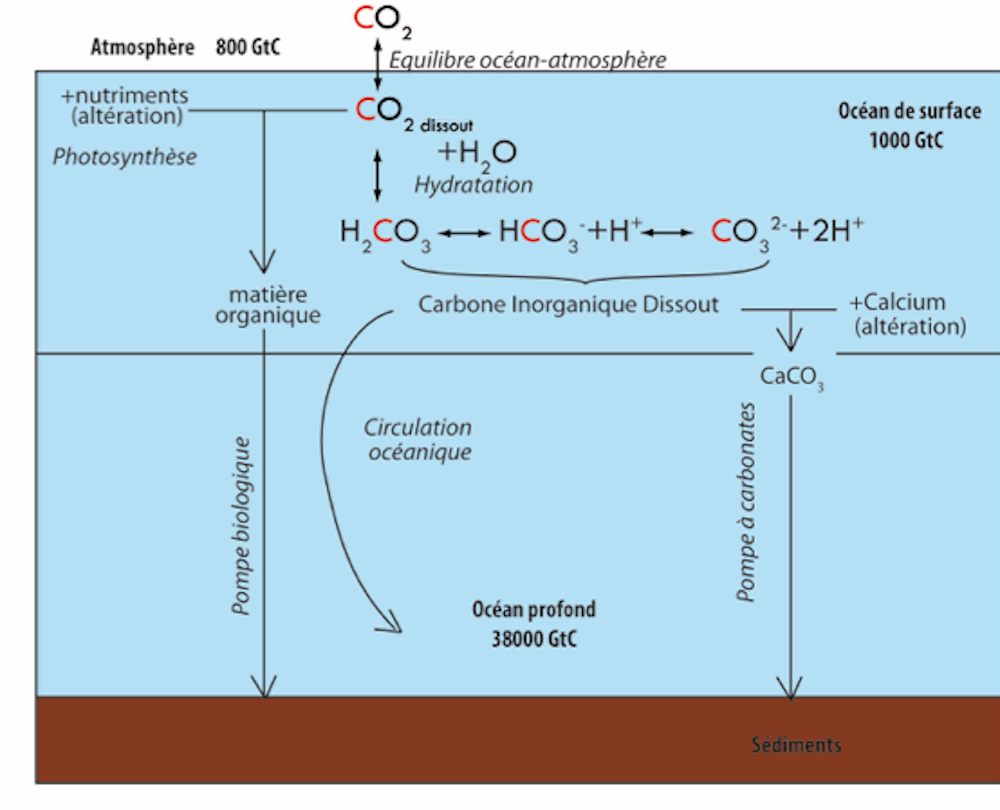
Source : Guillaume Paris
What causes ocean acidification ?
CO2 emissions are by far the main driver of ocean acidification. No need to go into details here, we already know what causes these emissions: human activities.
The second most important driver is agriculture, which emits large quantities of nitrogen compounds into the atmosphere, mainly N2O (nitrous oxide). When these nitrogen compounds dissolve in the ocean, they also have acidifying properties and contribute to ocean acidification. Compared to ocean acidification caused by rising CO2 emissions, the impact of the nitrogen cycle is rather small, but it’s raising more and more concerns, especially on coastlines where agricultural activities are important.
To a lesser extent, coastal pollution also leads to acidification, particularly through eutrophication, i.e. the excessive input of nutrients into the water which leads to plant proliferation, oxygen depletion and an imbalance in the ecosystem. This phenomenon is more local than the first two.
How has ocean acidification changed over time ?
This may be the only argument that climate deniers will use. “Oceanic acidity has already been high in the past. And humans have always adapted”.
A pH level at a given moment does not provide much information. However, its evolution is a crucial indicator. In less than 200 years, oceanic pH went from 8.2 to 8.1. This may not look like much, but make no mistake: the pH is logarithmic. Which means that a small difference of 0.1 corresponds to a 30% increase in oceanic acidity.
How can we observe this evolution ?
Sustained observations provide much needed data and understanding not only about ocean warming and water cycle reorganization (e.g., salinity changes), ocean eutrophication, and ocean deoxygenation, but also about changes in ocean chemistry.
To give an example: consistent variations in surface seawater CO2-carbonate chemistry are documented by seven independent CO2 time series that provide sustained ocean observations collected for periods from 15 to 30 years (see image above). In seven different parts of the world, the pH is consistently decreasing:
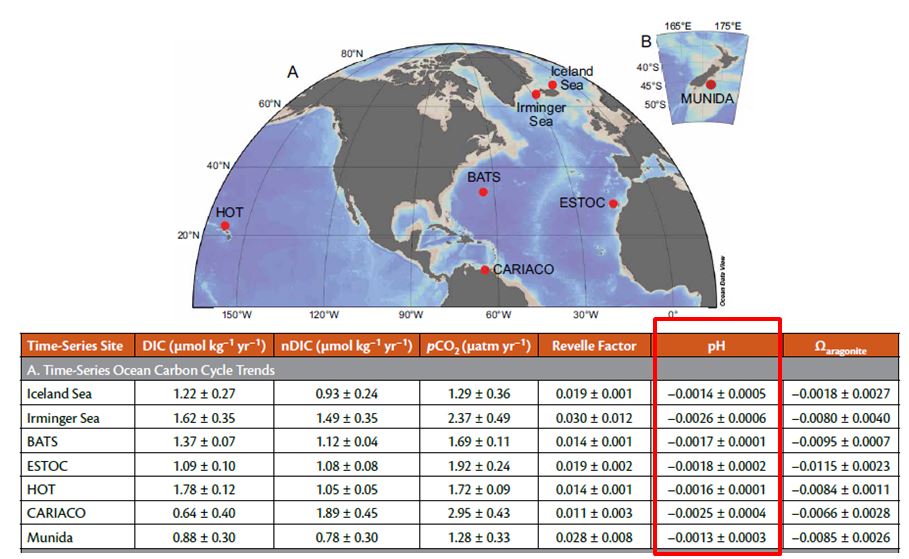
Source : Bates & al_2014
Has this already been observed in the past ? Yes. And yet, the alterations of the marine carbonate system observed since the industrial revolution are unprecedented in the last 65 million years. Is it a coincidence that this shift started when people began to burn coal on an industrial scale?
Here are 2 graphs: pay attention to the time scale. The first is expressed in millions of years:
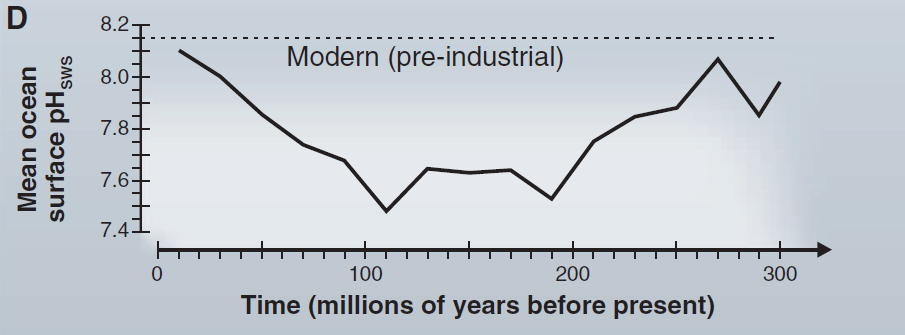
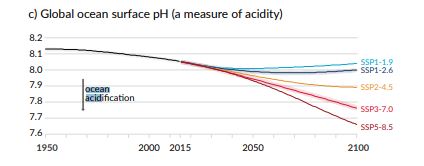
The second, in decades. The observed drop in pH is simply spectacular:
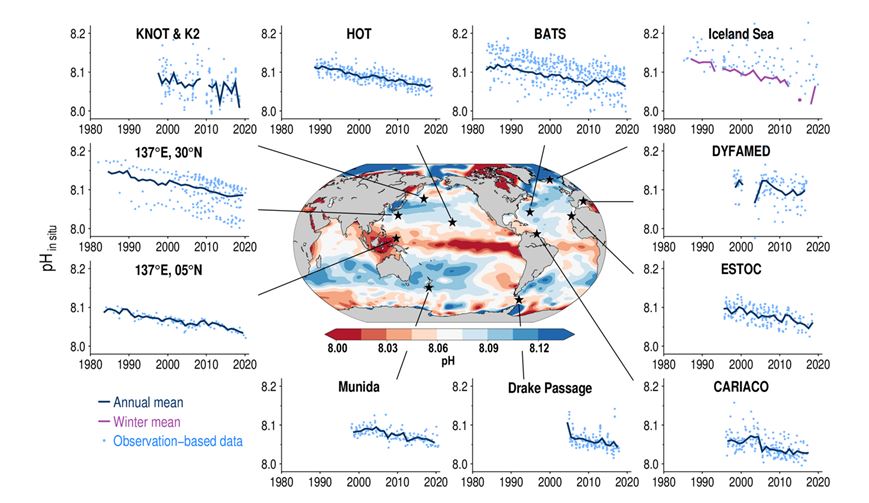
Source : AR6; Figure 5.20
How is past and present ocean acidification measured ?
We now know that oceans absorb carbon dioxide and that they are becoming more acidic (and pH is decreasing) as a result to oceanic measures taken during field missions.They provide broad spatial coverage over a short period of time as well as automatic or manual oceanic carbon measurements on stationary moorings, producing long-term, high-resolution data from a single location.
Up to a million years ago
These records can be extrapolated using so-called “proxies” that provide an indirect measure of marine carbonate chemistry. An indicator is a measurement taken from natural archive (ice cores, corals, tree rings, marine sediments, etc.) and is used to infer past environmental conditions.
For instance, by analyzing the chemical composition of tiny fossil shells found in deep ocean sediments, scientists have established records of oceanic pH levels in ancient times when pH measurers didn’t yet exist.
Also, because the chemical balance between surface water and the atmosphere is relatively stable, it’s possible to measure historical oceanic pH levels from atmospheric carbon dioxide concentrations. These are obtained from Greenland and Antarctic ice cores, which contain trapped air bubbles providing information on past climates. These data indicate that current atmospheric carbon dioxide concentrations and oceanic pH levels are unprecedented going back at least 800,000 years.
And even more ancient…
By going back even further in Earth’s history, to the Paleocene-Eocene boundary about 55 million years ago, scientists have found geochemical evidence of a massive release of carbon dioxide leading to a significant warming and dissolution of shallow carbonate sediments in the ocean.
Although similar to what we observe today, this release of carbon dioxide occurred during the course of thousands of years, at a much slower rate than what we are observing today, thereby giving the oceans time to partially adapt to the damage. Geological records show that, during periods of rapid environmental change, species have acclimated, adapted or disappeared. Corals have suffered mass extinctions in the past (such as the Permian extinction around 250 million years ago), and new coral species have evolved to take their place, but it has taken them millions of years to recover to previous biodiversity levels.
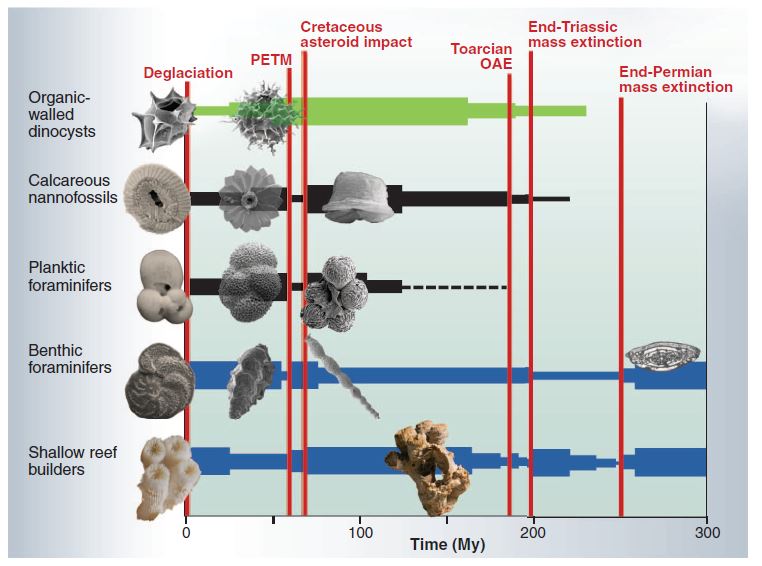
If there’s one lesson to learn,it’s that in the past, oxygen loss coupled with global warming and ocean acidification has systematically led to mass extinction. There were five of them, and wouldn’t it be great to avoid another one?
NEWSLETTER
Chaque vendredi, recevez un condensé de la semaine, des infographies, nos recos culturelles et des exclusivités.
+30 000 SONT DÉJÀ INSCRITS
Une alerte pour chaque article mis en ligne, et une lettre hebdo chaque vendredi, avec un condensé de la semaine, des infographies, nos recos culturelles et des exclusivités.
What role does ocean acidification play in the future ?
Thanks to the work of group 1 of the 6th IPCC report, we know that:
- “Under scenarios with increasing CO2 emissions, the ocean and land carbon sinks are projected to be less effective at slowing the accumulation of CO2 in the atmosphere.”
- “Upper ocean stratification, ocean acidification and ocean deoxygenation will continue to increase in the 21st century, at rates dependent on future emissions.”
- Global oceanic temperature changes (virtually certain) and deep ocean acidification are irreversible for decades or millenia (very high confidence).
- However, some of these changes can be slowed down or even stopped by limiting greenhouse gas emissions.
Future ocean acidification will therefore depend on our future emissions. This is indicated by the IPCC Special Report on Oceans and Cryosphere using the RCP scenarios, and more recently the AR6 using PSS scenarios. While the actual variation of -0.1 pH is already critical, a drop of -0.4 by 2100 is projected in worst case scenarios. This will have serious consequences for large parts of the oceans.
Is ocean acidification the same everywhere ?
Given that there are multiple drivers of ocean acidification, we can ask ourselves if its effects are the same everywhere in the world. There are some specificities to ocean acidification at three possible geographic scales:
- For instance, when focusing on a particular city and the impacts of waste water.
- Or at a regional scale, with sulfur pollution.
- On a global scale, there are no limits to CO2 spread.
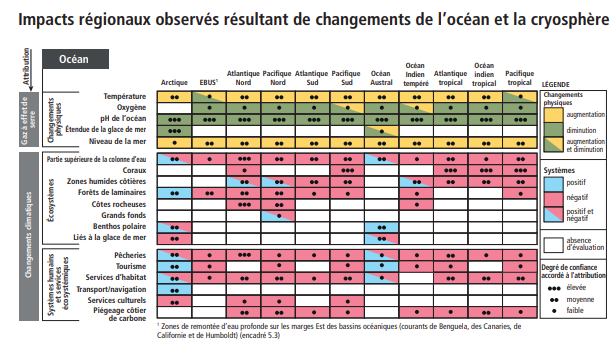
In the IPCC Special Report on Oceans and Cryosphere published in 2019, a graph showcases physical alterations at a regional scale. With a very high confidence degree, we find that oceanic pH will be decreasing in all of the world’s oceans.
Some regions could be more vulnerable to ocean acidification than others.
For instance,
- areas where deep, cold and low pH water rises back to the surface along continental plates shelves, on the west coast of North America for example.
- Or polar waters where low temperatures lead to higher CO2 absorption rates.
- and coastal areas where there are freshwater inputs.
Some volcanic areas also have their specificities. For example, on the island of Ischia in Italy, there are several sites called “champagne sites”, where CO2 emission are high which leads to locally higher oceanic acidity. These areas can be used as natural laboratories to understand the effects of ocean acidification on species, communities and ecosystems.

Credit : Maria-Cristina Gambi
In other words, they allow us to understand what will happen in the future given that, depending on the distance from the gas sources, the acidity there is comparable to what the world’s oceans will experience in the medium and long term.
What about France ?
If you think that ocean acidification is no concern of yours, I have some bad news.
Researchers have shown that the Northwestern Mediterranean sea went through rapid changes between 2007 and 2015. “The temperature increase there has been faster than anywhere else in the global ocean and acidification rates are among the highest measured values in the ocean. As a consequences, multiple organisms will be affected, which could alter the Mediterranean food chain“.
Between 2007 and 2017, sea surface temperatures increased by 0.7°C, which is much higher than in the global and coastal ocean. The pH decreased by 0.0028 units per year, which corresponds to an increase in acidity of 7%, one of the highest acidification rates recorded to date.
Of course, the combined effects of acidification and global warming are not without consequences regarding the contributions of Mediterranean ecosystems to human societies.
Consequences of ocean acidification
Research on the consequences of ocean acidification has never been as prolific as during the past 20 years. This could make for an entire article, given the sheer number of different consequences.
Consequences of marine life.
Let’s start with the obvious. Species do not all have the same adaptive capacity to changing environments. The oceanic chemistry is changing way too fast for a lot of species and populations to adapt through evolutionary changes. Here’s a non-exhaustive list of the consequences of ocean acidification on marine life:
- Changes in pH and carbonate chemistry require marine organisms to use more energy to maintain healthy body fluid chemistry.
- In some organisms, this change in energy expenditures could lower the energy allocated to other biological processes such as growth, reproduction, or the ability to respond to other stressors.
- Ocean acidification is already impacting marine life, especially organisms like oysters and corals that grow hard shells and skeletons by combining calcium and carbonate from seawater and are therefore highly sensitive to changes to ocean chemistry. In the following decades, we can expect very harmful conditions for corals, mollusks (oysters, clams, mussels …), pteropods (swimming snails) and some species of phytoplankton.

- Acidification can confuse fishes, interfering with their sense of smell and their behaviour. It can even change the way sound propagates underwater, making underwater seascapes slightly more noisy.
- Other non-calcifying organisms could also be stressed by elevated oceanic CO2 levels and by the decrease in pH levels and carbonate ions associated with ocean acidification.
- The impacts of ocean acidification on organisms at any life stage can decrease the ability of a population to develop or recover from stress-related damages.
Furthermore, the last IPCC report reminds us that consequences are not linear, but exponential. Scientists even warn that phenomena such as ocean acidification have the potential to trigger a number of abrupt changes or tipping points in the marine environment.

Consequences on the coastal population and the tourist economy
If ocean acidification affects biodiversity and aquaculture, it necessarily impacts human societies. Again, the scientific literature is crystal clear: the current trend will jeopardize the resources and services provided to populations that depend on the ocean.
The IPCC gives more information on that subject: “ocean warming and acidification corresponding to 1.5°C of global warming would impact a wide range of marine organisms and ecosystems, as well as sectors such as aquaculture and fisheries (high confidence)”. This warming will be observed in about ten years, not in 2050. Communities with close ties to coastal environments, small islands (including small insular developing states), polar regions and high mountain areas are particularly affected by oceanic changes. The consequences of such changes are harmful for indigenous peoples and local populations who live from fishing.
The disappearance of tropical reefs will jeopardize tourism, food security and coastal protection for many of the
world’s poorest societies. Fishing and aquaculture are already crucial aspects of global food security, and are already exposed to growing risks caused by ocean warming and acidification.
Climate justice and levers for action
Climate justice cannot be ignored when talking about such issues. The first human societies that will suffer from the consequences of ocean acidification are not the highest emitters of CO2. The levers for action are known and documented: we need to reduce our dependency on fossil fuels and stop massive deforestation. States that emit the most CO2 not only need to respect their engagements, but also help populations that will suffer the most to adapt.
In the Summary for Policymakers, the IPCC refers to anthropogenic CO2 removal (CDR) leading to net negative emissions globally, which would lower atmospheric CO2 concentration and reverse surface ocean acidification (high confidence).
But geoengineering methods that only aim to limit global warming through, for instance, a reduction of solar radiation, do not address ocean acidification, because they do not address the underlying cause of acidification (the increase of CO2 in the atmosphere) CO2 capture and storage can partially limit ocean acidification, but these techniques are currently only energetically and financially efficient on a small scale. In other words, if we want to avoid further biodiversity collapse and human disasters, we will have to rely on more than just technology.
The last word
- Ocean acidification is “A reduction in the pH of the ocean, accompanied by other chemical changes, over an extended period, typically decades or longer, which is caused primarily by uptake of carbon dioxide (CO2) from the atmosphere, but can also be caused by other chemical additions or subtractions from the ocean.”
- Since the eighties, the ocean absorbs over 25% of all anthropogenic CO2 emissions – around 40 billion metric tons of CO2– from the atmosphere each year.
- These CO2 emissions are by far the primary driver of ocean acidification. Then comes agriculture, and, to a lesser extent, coastal pollution, in particular because of eutrophication.
- In less than 200 years, oceanic pH went from 8.2 to 8.1. This means that a change of one tenth of a unit of pH — 0.1 — corresponds to a 30% increase in oceanic acidity.
- Ocean acidification (very high confidence) and ocean deoxygenation (high confidence) will continue to increase during the course of the 21st century, the intensity of this increase will depend on future emissions.
- In the past, oxygen loss coupled with global warming and ocean acidification has consistently led to mass extinction.
- The widespread loss of tropical coral reefs will jeopardize food security, tourism and coastal protection for many humans, disproportionately affecting the world’s poorest countries.